INTRODUCTION
Pigment dispersion syndrome (PDS) is a unique
and fascinating entity. Far more prevalent than previously suspected, (89) it is the first common disease leading to glaucoma for
which we are on the verge of a coherent overall explanation of pathogenesis and
pathophysiology. This paper is an attempt to tie together many interesting and sometimes
disparate and/or apparently anomalous findings in order to synthesize a coherent portrait
of the disease.
This is the beginning of a Living Document on
PDS and pigmentary glaucoma (PG). The concept of a living document is to create a summary
and databank of all the world's knowledge on this particular subject. It will grow and
develop over time. Ideally, in the future, newly discovered facts may be peer reviewed and
inserted directly into the Document. This Document is intended to serve as a source of
information both for professionals and patients. As such, it may be highly technical to
some readers. A glossary is being developed and will be posted when completed.
Nevertheless, with the extensive illustrations, the gist of the material should be largely
intelligible to the interested reader.
PDS and pigmentary glaucoma (PG) are
characterized by disruption of the iris pigment epithelium (IPE) and deposition of the
dispersed pigment granules throughout the anterior segment. The classic diagnostic triad
consists of corneal pigmentation (Krukenberg spindle; slit-like, radial, mid-peripheral
iris transillumination defects, and dense trabecular pigmentation. The iris insertion is
typically posterior and the peripheral iris tends to have a concave configuration. The
basic abnormality in this hereditary disorder remains unknown.
Back
HISTORY
In 1899, Krukenberg (56) described spindle-shaped pigment deposition on the
cornea. In 1901, von Hippel (111) suggested that pigment
obstructing the aqueous outflow system could lead to elevated intraocular pressure (IOP).
Levinsohn (59) first suggested that pigment in the
anterior chamber angle of patients with glaucoma originated from the IPE. A
cause-and-effect relationship between pigment and glaucoma found both support (46,51) and opposition (12,30,110).
In 1949, Sugar and Barbour (107) described two young, myopic men with Krukenberg
spindles, trabecular hyperpigmentation and open angles, whose IOP increased with mydriasis
and decreased with pilocarpine. They identified the disorder as a rare, distinct form of
glaucoma, which they termed pigmentary glaucoma. More patients were subsequently reported,
and in 1966 Sugar (106) reviewed 147 cases in the world
literature, mentioning several additional features, including bilaterality, frequent
association with myopia, greater incidence in men than in women, and a relatively young
age of onset. These features were confirmed by Scheie and Cameron. (94)
In the 1950s, the discovery of iris
transillumination defects led to the concept that the trabecular pigment originated from
the IPE and perhaps the ciliary body. (10,95) Congenital atrophy or degeneration of the IPE was
suggested as a cause of loss of iris pigment.(14,91)
In 1979, Campbell (18) proposed the pathogenesis to involve mechanical damage
to the IPE during rubbing of the posterior iris against the anterior zonular bundles
during physiologic pupillary movement. Subsequently, the autosomal dominant inheritance,
natural history, reversibility, and more precise therapeutic approaches have become
increasingly delineated. Ultrasound biomicroscopic studies are presently revealing new
insights into the pathophysiology of PDS.
Back
CLINICAL
FINDINGS
A. ANTERIOR SEGMENT
Loss of iris pigment appears clinically as a
midperipheral, radial, slit-like pattern of transillumination defects seen most commonly
inferonasally and more easily in blue eyes than in brown ones. Although the defects can
sometimes be seen by retroillumination, they are more easily detected by a dark adapted
examiner using a fiberoptic transilluminator in a darkened room. Infrared videography
provides the most sensitive method of detection.(3)
Pigment particles deposited on the iris surface tend to aggregate in the furrows.(76,106) Rarely, this
pigment can be dense enough to darken the iris or to cause heterochromia when involvement
is asymmetric.(60,106)
Iris vascular hypoperfusion on fluorescein angiography has been reported,(36) a finding which awaits verification.
Anisocoria may occur with asymmetric
involvement, the larger pupil corresponding to the eye with greater pigment loss from the
iris.(2,31,32) Alward and Haynes (2)
suggested the presence of an efferent defect in the eye with the larger pupil. The pupil
may be distorted in the direction of maximal iris transillumination.(31,32,42) This would be consistent with the presence of
hyperplasia of the iris dilator muscle (see below).(40)
Corneal endothelial pigment generally appears
as a central, vertical, brown band (Krukenberg spindle), the shape being attributed to
aqueous convection currents. The pigment is phagocytosed by endothelial cells,(43,52) but endothelial cell
density and corneal thickness remain unchanged compared to controls.(76) Coincident PDS and megalocornea has been reported,(17,91,94,100) as have subluxated
lenses.(88,94)
The anterior chamber is deeper both centrally
and peripherally than can be accounted for by sex, age, and refractive error. Davidson (et
al. 25) compared the central and peripheral anterior
chamber depths of patients with PDS to statistical controls. The anterior chamber was
significantly deeper and the anterior chamber volume was significantly greater in the PDS
group, the difference being greatest inferiorly.
The angle is characteristically widely open,
with a homogeneous, dense hyperpigmented band on the trabecular meshwork. Pigment may also
be deposited on Schwalbe's line. The iris insertion is posterior and the peripheral iris
approach is often concave. The iris is most concave in the midperiphery. In younger
patients, the scleral spur may be poorly demarcated, blending with the ciliary face due to
pigment deposition on these structures. Pigment may be deposited on the zonules (60,95,114) and on the posterior capsule of the lens, where it is
apposed to the anterior hyaloid face at the insertion of the posterior zonular fibers.(8,50,95,114)
Back
Figure 1. Krukenberg spindle.
Liberated pigment granules are borne by aqueous currents and deposited on the structures
of the anterior segment. The vertical accumulation of thesepigment granules along the
corneal endothelium is known as Krukenberg's spindle). The spindle tends to be slightly
decentered inferiorly and wider at its base than its apex
|
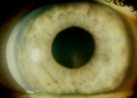 |
Figure 2. Ultrasound
biomicroscopy in PDS.
The iris concavity in PDS has been investigated using high frequency, high resolution
ultrasound biomicroscopy. Ultrasound biomicroscopy is an innovative diagnostic tool which
employs high frequency ultrasound to permit high resolution in vivo imaging of the
anterior segment. It has been particularly useful in the evaluation of the structures
surrounding the posterior chamber. The iris (I) is bowed posteriorly, towards the zonules
and posterior chamber (PC). The ciliary body (CB), cornea (C), anterior chamber (AC), and
lens capsule (LC) are visible. Although most young individuals with undisputed PDS (young
age, zonular pigment dispersion, increased meshwork pigmentation, myopia) have a
demonstrable iris concavity which can be measured during ultrasound biomicroscopy, the
prevalence of iris concavity at the time of initial diagnosis has not been evaluated in a
large study.
|
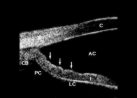 |
Figure 3. Iris
transillumination.
Movement of the posteriorly bowed, concave iris along the anterior zonular bundles causes
a disruption of the iris pigment epithelium along the radial orientation of the zonular
fibers which results in characteristic mid-peripheral, iris transillumination defects seen
during slit-lamp examination. This finding is pathognomonic for zonular pigment dispersion
and differentiates PDS from other glaucomas related to accumulation of pigment in the
trabecular meshwork.
The width, length, and frequency of these defects varies among individuals and a high
index of suspicion on the part of the examiner is often needed to make the diagnosis. It
is best to search for iris transillumination defects prior to pupillary dilation by using
a small slit beam in a darkened room. However, those patients who do not appear to have
transillumination defects on retroillumination but have increased trabecular pigmentation,
Krukenberg spindle, myopia or juvenile open angle glaucoma should be examined with scleral
transillumination using a fiberoptic scleral transilluminator in a darkened room to
facilitate detection. Pupillary dilation may prevent the detection of transillumination
defects because of the compaction of the peripheral iris stroma.
|
 |
Figure 4. Infrared video
pupillography.
The number of iris transillumination defects often corresponds clinically to the degree of
anterior segment pigment liberation and elevated IOP, although this is not always the
case. In eyes with asymmetric disease, the eye with the higher pressure is invariably the
one with the greater pigment liberation.Some physicians have advocated the documentation
of the numbers of transillumination defects as a means of the following the progression of
the disease. Individuals in the pigment liberation phase of the disease typically have an
increasing number of transillumination defects, whereas those individuals who are no
longer actively liberating pigment may have defects which shrink in size or disappear.
Although standard slit-lamp photography can be used to document the number of defects,
infrared video pupillography may provide more accurate visualization
|
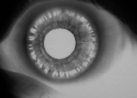 |
Figure 5. Iris surface
pigmentation.
Pigment accumulation on the anterior surface of the iris often appears as concentric rings
within the iris furrows. More diffuse pigmentation can cause a diffuse darkening of iris
color, which is more apparent in lightly pigmented irides because of the degree of color
change. Asymmetry of pigment liberation may result in iris heterochromia, with the darker
iris being the more affected side.
|
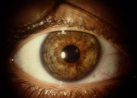 |
Figure 6. Trabecular
pigmentation.
Increased trabecular pigmentation occurs in a wide variety of glaucomas. In PDS, the
trabecular pigmentation is typically homogeneous in its distribution, unlike the
variegated appearance associated with exfoliation syndrome, uveitis, or angle-closure
glaucoma. The degree of pigmentation ranges from moderate to dense and is often quite
striking. In some individuals the increased pigmentation may be limited to the posterior
trabecular meshwork, while in others the anterior meshwork, Schwalbe's line, or peripheral
cornea may be covered with dense pigmentation.
|
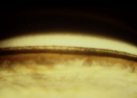 |
Figure 7. Lens
pigmentation.
Pigment deposition on the zonular apparatus may allow visualization of the radial anterior
zonules as they traverse the posterior chamber to the anterior lens surface. Since
liberated pigment floats freely within the aqueous, some of the pigment granules may also
move posteriorly behind the lens equator, where they accumulate at Weigert's ligament, the
region of contact between the anterior hyaloid face and the posterior lens capsule.
Visualization of this circular ring or arc of pigmentation requires pupillary dilation and
upon occasion, gonioscopy, and is considered pathognomonic for PDS, since it has not been
identified in other disorders associated with pigment liberation in the anterior segment.
|
 |