CLINICAL CORRELATIONS
A. HEREDITY
The above concept of the pathophysiology of
PDS helps us to better understand a number of clinical aspects of the disorder. Structural
abnormalities are characteristic of autosomal dominant disorders. Only occasional families
with Krukenberg spindles were reported prior to the 1980s. (70,92,94,97,103,105,110) Reports in the
19801s described familial PDS, but were inconclusive regarding the mode of inheritance. (44,47,68,77) McDermott et al (72) examined relatives of 21 probands, and found involvement
in 36% of parents and 50% of siblings, but none in children under the age of 21 years.
This suggested a strong pattern of autosomal dominance, with phenotypic onset probably
beginning in most persons in the mid-20s. That Caucasians are almost exclusively affected
is also consistent with a genetic origin.
Back
B. GENDER
Men and women are equally affected by PDS,
women having predominated in some series (11,94) and men in others. (61,66) However, men develop glaucoma about 3 times as often as
women and at a younger mean age. (6,66,74,94,106) Berger et al (9) found no difference in age at diagnosis of PDS between men
and women, but men were significantly younger than women at the time of diagnosis of PG.
No population based study has yet been performed. If myopia is the major determinant of
phenotypic expression, then one would expect an equal incidence of men and women, since
the prevalence of myopia in the United States is similar between men and women. (102) Why then do more men develop glaucoma and do women
appear to develop it at a somewhat older mean age? It is possible that female hormones
exert a protective effect against the development of elevated IOP. A curious and
unconfirmed finding reported by Duncan (27) was the
development of Krukenberg spindles in 4 black women during pregnancy; these regressed
after delivery. One report relating to hormonal treatment of PG has never received further
attention in the literature. (71)
Back
C. REFRACTIVE
ERROR
About 60-80% of patients with PDS and PG are
myopes and 20% are emmetropes (-1.00 to +1.00 diopters). (94,106) In earlier series which reported about 10% of patients
to be hyperopes, there appears to have been some confusion between PDS and exfoliation
syndrome, particularly as the hyperopes in these series tended to be older and to be
women. Eyes with PG are significantly more myopic than those with PDS and the higher the
myopia, the earlier is the age of onset of glaucoma. (9)
Campbell (18,19) suggested that enlargement of the myopic eye in young
patients allows the peripheral iris more space in which to bow posteriorly. Kaiser-Kupfer
et al (47) mentioned that transillumination defects can
precede the development of myopia and increase without any concomitant progression of
significant refractive error.
Back
D. ASYMMETRIC
INVOLVEMENT
Since PDS is a bilateral disorder, asymmetric
involvement requires explanation. A second disorder may make one eye worse. The most
common cause in older patients appears to be the development of exfoliation syndrome in
one eye in patients who had had PDS or PG glaucoma in earlier life. (58) Angle recession in one eye has also been reported. (85) It is also possible for one eye to have a second
disorder which reduces the severity of PDS, such as unilateral traumatic cataract
extraction in youth prior to the onset of pigment dispersion or development of unilateral
cataract during the pigment dispersion phase, which decreases iridozonular contact by
causing pupillary block. (90) Horner's syndrome may
achieve the same effect. (54) We have also seen
anisometropic patients with greater involvement in the more myopic eye (unpublished data).
In other cases, mild to marked asymmetry may
exist without any other evident process. Kaiser-Kupfer et al (47)
reported 4 normotensive patients with markedly asymmetric involvement and no obvious cause
for asymmetry. Three had anterior chamber depths 0.2 mm greater in the affected eye.
Anderson (4) remarked that there should be asymmetry in
the anatomic or physiologic factors relevant to the underlying pathogenesis. Liebmann et
al (63) examined four patients with markedly asymmetric
PDS and no other ocular conditions to explain the asymmetry and found greater
iridolenticular contact and a more posterior iris insertion in the more involved eye in
all cases.
Back
A. ACTIVE PHASE
The mean age of onset of PDS remains unknown,
but is probably in the mid-20s. The youngest patients reported have been aged twelve, (47) fourteen, (9,94) and fifteen. (91)
Although it seems logical that PDS might develop in the mid-teens, when myopia is commonly
progressive, a screening of over 300 students at Stuyvesant High School, a school for
especially intelligent children in New York City, did not reveal a single case
(unpublished results). Moreover, McDermott et al (72)
found no children of probands positive up to age 21. Further studies are warranted. The
development of PDS later in life is unlikely because of gradual lens enlargement and loss
of accommodation.
The phenotypic expression of PDS varies
widely. Referral practices tend to have patients with more extensive involvement, although
even in these patients, the diagnosis is often missed. More subtle manifestations may
never be detected either because of a lack of suspicion on the part of the examiner,
unawareness of the examiner of pathognomonic signs in patients with mild phenotypic
involvement, failure to perform slit-lamp examination in patients presenting for
refraction, and simply lack of an eye examination. Failure to perform gonioscopy may
result in lack of diagnosis of patients with trabecular hyperpigmentation but without
Krukenberg spindles, since transscleral transillumination is often the least likely test
to be performed. It is not known whether the variability in phenotypic expression is
hereditary, environmental, or a combination of both. For instance, the concavity due to
iris position and size (genetic) could be affected by the cumulative amount of
accommodation (environmental). Further studies are warranted.
Back
B. REGRESSION
PHASE
The timing of the onset of the regression
phase of PDS is easier to explain. The severity of involvement of both PDS and PG
decreases in middle age when pigment liberation ceases, at least in the majority of
patients. Lichter and Shaffer (61) observed decreased
pigment in the trabecular meshwork in 10% of 102 cases, concluding that pigment could pass
out of the meshwork with age. Transillumination defects may disappear, (18,28) most likely
by migration of pigment epithelial cells adjacent to the defects. The IOP may return
toward normal. (28,101,113) Some patients treated with long-term miotic therapy
have been able to reduce or discontinue treatment for glaucoma. (20,101) Older patients
presenting with glaucoma may have only very subtle manifestations, if any, of PDS, and may
be misdiagnosed as primary open-angle glaucoma or low-tension glaucoma.(84) Remission of PG has also been reported following
glaucoma surgery (94) and following lens subluxation. (88)
Trabecular pigmentation is initially dense
and homogeneous for 360 degrees. With age and clearance of pigment from the angle, it
becomes lighter and more localized to the filtering portion of the meshwork, while it
disappears from Schwalbe's line and the scleral spur. When the trabecular meshwork begins
to recover, the normal pigment pattern reverses and the pigment band becomes darker
superiorly than inferiorly. We have termed this the "pigment reversal sign" and,
in older patients, it may be the only finding suggestive of previous PDS. Although it
cannot be regarded as diagnostic, examination of the patient's offspring in such a case
may be confirmatory. The pigment reversal sign may also be found in patients after
long-term miotic therapy in patients with PDS/PG and also in patients with exfoliation
syndrome, confirming that it occurs as a result of pigment clearing from the meshwork.
Back
C. LOGIC OF TREATMENT
The development of relative pupillary block
secondary to an age-related increase in lens thickness and loss of accommodation with the
onset of presbyopia are two processes which presumably lead to the cessation of pigment
liberation in middle age. Older patients with PDS develop little or no accentuation of the
iris concavity with accommodation. (81) By eliminating
the iris concavity and iridozonular contact, miotic therapy may prevent progression of the
disease and the development of glaucoma by immobilizing the pupil and may allow previously
existing damage to reverse more readily. Since most PDS patients are young and cannot
tolerate pilocarpine drops because of induced myopia and accommodative spasm, pilocarpine
Ocuserts have proven to be the best available for of miotic therapy.
The success rate of argon laser
trabeculoplasty (ALT) in PG is greater in younger patients than in older ones and
decreases with age. (62,67,87) Pigment in younger patients is largely in the
uveoscleral and corneoscleral meshworks, whereas in older patients, it if primarily
localized to the juxtacanalicular meshwork and the back wall of Schlemm's canal. (87) A larger portion of patients fail within a shorter
period of time compared to POAG patients. (38,67,87) Initially successful
trabeculoplasty may be followed by a sudden, late rise in IOP, similar to that seen in
exfoliative glaucoma. Patients in the pigment liberation stage who undergo ALT should be
maintained on miotics or undergo laser iridotomy after ALT to prevent further contact
between the iris and zonules. Although topical miotic drops or gel preparations are poorly
tolerated by younger patients due to induced myopia and accommodative spasm, pilocarpine
Ocuserts are extremely well tolerated.
Back
MANAGEMENT
Since the degree and stage of pigment
liberation, intraocular pressure, and extent of glaucomatous optic neuropathy vary among
individuals, each must be evaluated to determine the proper course of intervention. As our
understanding of the pathogenesis of pigment liberation expands, consideration should also
be given to gearing therapy towards eliminating acute pigment release, rather than just
treating elevated IOP.
Beta-adrenergic antagonists. The
mainstay of initial medical therapy for PG continues to be aqueous suppression with a
topical beta-blocker, primarily because of the relatively easy dosing schedule and minimal
side effects.
Parasympathomimetics. In theory,
therapy directed at increasing relative pupillary block should relieve iridozonular
contact and diminish pigment liberation. The relief of iridozonular contact following
miotic therapy has been demonstrated with ultrasound biomicroscopy (Figure 15, 16).
However, strong miotics in young individuals are rarely tolerated because of the
associated spasm of accommodation and blurring of vision. Low-dose pilocarpine in the form
of Ocuserts often provide enough miosis to create pupillary block, without disabling
adverse effects. A careful peripheral retinal examination should be performed before and
after the institution of or change in miotic therapy because of the higher incidence of
retinal breaks and detachment in these patients.
SURGERY
Laser trabeculoplasty. Argon laser
trabeculoplasty may be offered as a treatment in the management of uncontrolled PG.
Although the initial result is often good, a larger proportion of patients can lose
control of IOP when compared to primary open angle glaucoma patients, and the loss of
control can occur in less time. In contrast to other forms of open angle glaucoma, younger
patients appear to respond better to trabeculoplasty than do older individuals.
Laser iridectomy. Laser iridectomy
eliminates the iris concavity present in PDS by permitting equalization of pressures
between the anterior and posterior chambers. This causes the iris to become flat, thereby
decreasing iridozonular contact (Figure 17). Anecdotal evidence suggests that this can
prevent continued pigment liberation, result in a reversal of trabecular pigmentation, and
subsequently, lowering of IOP. Although this approach is theoretically sound, laser
iridectomy should be used with caution because there is a paucity of data regarding the
long-term efficacy of this procedure.
Back
Figure 22.
Pre-laser iridotomy scan demonstrating marked iris concavity and central iris contact with
anterior lens capsule and zonules. |
 |
Figure 23.
Post-laser iridotomy scan shows resolution of iris concavity and decreased length of iris
contact with anterior lens capsule. |
 |
Figure 24.
Repeat scan eleven days later shows recurrence of iris concavity. Slit lamp evaluation had
demonstrated occlusion of the laser iridotomy by pigment. |
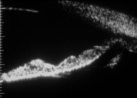 |
Figure 25.
Concavity resolved after reopening laser iridotomy. |
 |
Figure 26.
Prior to laser iridectomy, gonioscopy demonstrates a concave iris configuration. |
 |
Figure 27.
Following laser iridectomy, the iris assumes a flat configuration. |
 |
THE
BASIC LESION
Any hypothesis concerning the basic defect in
PDS must take into account the various anatomic findings noted above. Most difficult is
explaining the relationship to lattice degeneration. A structural abnormality of the
middle third of the eye causing an abnormally concave peripheral iris and the vitreous
base/anterior retina to be drawn anteriorly could be consistent with previously proposed
mechanisms.
During the formation of the secondary
vitreous, a condensation of fibers extends laterally between the lens and the iris to form
the marginal bundle of Druault, which extends backward between the lens periphery and the
equator, attaching strongly to the internal limiting membrane of the peripheral retina to
form the vitreous base. (5) It also attaches to the
posterior capsule of the lens around the primary vitreous, as a ring 8-10 mm in diameter,
to form the hyaloideocapsular ligament of Wiegert. Developing zonular fibers (tertiary
vitreous) pass through this bundle at right angles. As the ciliary processes and the iris
develop, the marginal bundle loses its connection anteriorly, but remains attached to the
peripheral retina at the vitreous base. (5) A condensation
of the anterior surface of the secondary vitreous finally separates the zonular fibers
from the vitreous. An abnormal persistence of connections between the zonular apparatus
and the marginal bundle of Druault might lead to tension on the peripheral retina.
During the 7th month, the apex of the angle
moves posteriorly to become level with the middle portion of the meshwork. This is due not
to cleavage, but to a differential growth rate of anterior neuroectoderm and anterior
periocular mesenchyme, the latter growing more rapidly. (5)
The ciliary processes move backward and become located behind the apex of the angle.
The responsible gene should also influence
the size of the iris relative to the anterior segment and perhaps the susceptibility of
the IPE to disruption by zonular friction. A gene affecting some aspect of the development
of the middle third of the eye early in the third trimester appears reasonable at the
present time.
Back
SUMMARY
In sum, PDS is an
inherited disorder of abnormal iridozonular contact which is exaggerated by physiologic
pupillary movement and accommodation. This contact results in disruption of the IPE cells
and liberation of pigment, which is deposited on structures throughout the anterior
segment. Pigment liberation can be triggered by exercise and by pupillary dilation. Myopia
predisposes to the phenotypic expression of the disorder, which affects men and women
equally, but men develop glaucoma 2-3 times as often as women and at an earlier age.
Pigment dispersion begins in the teens or twenties and continues until about the mid-40s
in most people, at which time a combination of relative pupillary block and presbyopia
lead to gradual cessation of pigment liberation. After this, the visible signs of pigment
loss can reverse and IOP control can improve. Older patients presenting for the first time
with glaucomatous damage and normal IOP may be misdiagnosed as having normal-tension
glaucoma.
Anatomically, the iris seems excessively
large for the eye and is posteriorly inserted, resulting in a characteristic concave
midperipheral configuration, iridozonular contact, and abnormally extensive
iridolenticular contact. When blinking is inhibited, the iris assumes a convex
configuration which is immediately reversed upon blinking, suggesting that the act of
blinking acts as a mechanical pump to push aqueous humor from the posterior to the
anterior chamber. Once in the anterior chamber, aqueous backflow is prevented by the
abnormal iridolenticular contact, which produces a reverse pupillary block, further
enhancing the iris concavity.
Treatment should begin early in order to
prevent the development of glaucomatous damage and should be designed to prevent
progression of the disease rather than merely lower IOP. Miotic treatment produces a
convex iris configuration, completely inhibiting pigment liberation, while laser iridotomy
produces a planar configuration and may not completely inhibit pigment liberation. Aqueous
suppressants theoretically may negatively impact the course of the disease. Argon laser
trabeculoplasty produces better results in younger patients than older ones because of the
location of the pigment in the trabecular meshwork.
Persons with pigment dispersion also have an
abnormally high incidence of lattice degeneration of the retina and retinal detachment.
Any hypothesis regarding the origin of this disease must take this into account. It must
also provide a reason why many myopes without PDS have an iris concavity which also
increases with accommodation. An abnormal persistence of the marginal bundle of Druault
might lead to an abnormality of zonular position. The responsible gene should also affect
the size of the iris and perhaps susceptibility of the IPE cells to disruption. A gene
affecting some aspect of the development of the middle third of the eye early in the third
trimester appears at the present time to be the most likely cause.